i2medical LLC (hereinafter i2medical), jointly with the Keio University School of Medicine, is investigating and developing a “digital medical device for aiding depression diagnosis” that enables screening for depression and rating of severity only by means of a wearable device. This technology has already been patented and is under a commercialization project entitled “SWIFT,” jointly with Sumitomo Dainippon Pharma Co., Ltd. (hereinafter Sumitomo Dainippon Pharma).
In “SWIFT,” the team is developing a system for collecting vital sign and activity data using a wristband-type device, and analyzing the data by means of a machine learning algorithm to detect the patient’s depressive symptoms and estimate the severity of depression, thus creating a structure to provide information that serves as rating material for the physician to make a diagnosis.
Depression is known as a disease of which the condition is difficult to understand accurately, for both the patient and attending physician. If a communication tool for objectively describing depressive symptoms is created, the present status of depression in the social environment will change dramatically. Depression is said to be a “common cold of mentality,” and can be managed appropriately if recognized before increasing in severity. The following is an overview of the “SWIFT” project aiming at such an environment.
Present Status of Depression in Japan and Difficulty on Measuring Objective Data
The lifetime prevalence rate of depression in Japan is 5.7%*1. The social cost is said to amount to 3.1 trillion yen per year*2.
Depression poses a major concern in areas of industrial health, because it is prevalent among people of working age groups. It is a key issue for employing companies to prevent the onset and post-reinstatement recurrences of depression; the recent increasing trends for the disease, along with the influence of the spread of COVID-19, have been of growing concern worldwide.
However, clinicians for depression and other psychiatric diseases are facing difficulty, as “the absence of effective objective indicators hampers their evaluations.” The situation is currently coped with by scoring mood, sleep, appetite, and other factors based on interviews and questionnaires in clinical settings; however, the patient’s subjective views cannot be eliminated, and thus poor objectivity and rating inconsistency among the evaluators are unavoidable. This leads to a problem with difficulty in providing uniform quality of medical services.
In depression, for example, symptoms of slower thinking are generically referred to as an “inhibition of thought.” This can also influence activities, thus making it possible to objectively observe the content and speed of a patient’s responses. Therefore, “inhibition of thought” is deemed an indicator that can be evaluated somewhat objectively in the diagnosis of depression.
However, there are no established criteria for defining the term “inhibition of thought” as a symptom of depression in relation to the patient’s response speed. Therefore, an evaluation to determine “the presence or absence of inhibition of thought” unavoidably depends to some extent on the psychiatrist’s subjective view. Such limitations on objective evaluations and difficulty with accurate understanding of mental condition underly the cause of the difficulty with treatment of depression.
In addition, depression in its early stage is said to be difficult for untrained non-psychiatric physicians to diagnose; there are some cases in which the patient is referred to a department of psychiatry too late after presenting with physical complaints or insomnia. Depression sometimes fails to be evaluated in busy medical settings, and this raises the risk of exacerbation of the condition until the appropriate treatment is delivered to the patient.
“SWIFT System” for Objective Evaluations of Depression
Aiming to Develop Medical Devices that Enable Screening of Depression and Rating of Severity by Means of a Wristband-Type Wearable Device

The joint “SWIFT” project being undertaken by i2medical, Keio University School of Medicine, and Sumitomo Dainippon Pharma aims to develop a wristband-type medical device for screening for depression and rating of severity simply by it being worn by a patient.
This project aims to provide a tool for objective evaluation of depressive symptoms and communication in a common language to improve the quality of communication among patients, physicians, specialists, and nonspecialists, thereby helping the sufferers of depression.
The use of the “SWIFT system” is expected to provide objective and quantitative information on depressive symptoms. It is considered that the ability to manage depression at the discretion of physicians based on medical judgments that come from objective indicators will facilitate the early detection and treatment of the disease, hence leading to the creation of a sound society in which everyone can enjoy an active life.
In recent years, technical advances in IoT and microsensors have made it possible to continuously collect a broad range of biological data, including action volume, sleep, and other activity data, as well as heart beat fluctuations, pulse rate, and skin temperature, in daily life settings.
The “SWIFT system” aims to provide objective indicators for diagnosis of depression by comprehensively evaluating various vital sign and activity data that may indicate depressive symptoms communicated via a set of sensors equipped on the device, which have been difficult to evaluate using a single index so far.
Furthermore, the recent advances in machine learning technology based on artificial intelligence (AI) have enabled us to build a complex predictive model by simultaneously using a very large number of indicators. This has made it possible to make analyses based on larger amounts of data than ever before, offering a potential for highly accurate screening for depression and rating of severity.
The device used in the “SWIFT system” is a wristwatch-type wearable device. In addition to an acceleration sensor, pulse rate sensor, and temperature sensor, the device is equipped with an ultraviolet sensor and microphone, the data from which can be used to calculate a wide variety of parameters; for example, acceleration rates, number of walking steps, calorific consumption, speech volume, sleep time, quality of sleep, and pulse rate.
The data measured by the device is automatically uploaded to a cloud storage system, where they are analyzed and transferred to a medical institution. At the medical institution, the data will be used as objective indicators for diagnosis by a psychiatrist, as basis for rating of therapeutic effects and choice of treatment, and even as an aid to identify recurrence signs and determine the timing of work suspension and reinstatement. It is also likely that nonspecialists who do not belong to the department of psychiatry will use the “SWIFT system” as a guide to referrals of their patients to specialists.
Furthermore, objective indicators from the “SWIFT system” may be used to determine therapeutic effects, which in turn can optimize the treatment. Other expected uses include determinations of the necessity for therapeutic switches on a short-time basis, and monitoring of changes in the patient’s depressive symptoms on a long-term basis.
Toward Commercialization Making the Best Use of Patented Technology through Industry-Academy Collaboration

The “SWIFT” project is being implemented by a tripartite team of i2medical, Keio University School of Medicine, and Sumitomo Dainippon Pharma. In the background of this collaboration, Taishiro Kishimoto, President of i2medical, is serving concurrently as a lecturer at the Department of Neuropsychiatry of Keio University School of Medicine, and as a Project Professor at the Hills Joint Research Laboratory for Future Preventive Medicine and Wellness in the School of Medicine, Keio University, as of 2022.
Setting its mission to resolve indeterminate issues in psychiatry through innovation by a fusion of psychiatry with multiple technically advanced disciplines, his laboratory has been working on evaluations and treatments of psychiatric diseases by means of natural language processing, machine learning, and advanced technologies for sensing, remote healthcare, and so on.
i2medical was founded mainly by the members of the laboratory. This challenge arose from the belief that the best way to speed social applications of research achievements was to commercialize the achievements by themselves.
In the current “SWIFT” project, i2medical is playing a role in developing an algorithm for depression evaluations by machine learning, which is the “heart” of the “SWIFT system,” and increasing the accuracy, by making the best use of the knowledge of psychiatrists and in-house data scientists.
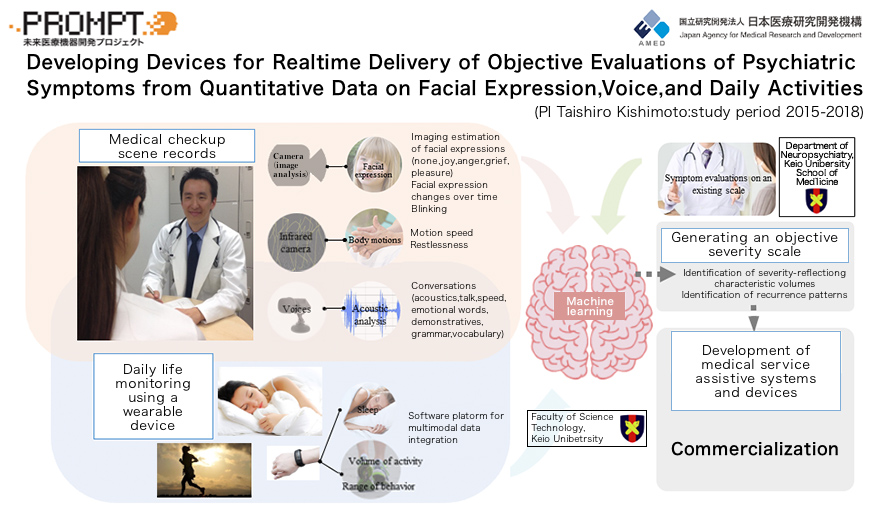
A research project we called “PROMPT” served as a prototype for the “SWIFT” project with Sumitomo Dainippon Pharma. i2medical has been conducting research on evaluation of depression and so on by machine learning with the use of facial expression, attitude, and voice data from cameras and microphones, as well as daily activity monitoring of data from wearable devices. The joint development with Sumitomo Dainippon Pharma began when this technology became patented.
Future Prospects for “SWIFT”
The “SWIFT system” is expected to find applications and advances not only in clinical settings for depression, but also in other research domains. For example, a combination of objective indicators from the wearable device and machine learning is considered to make it possible to distinguish between patients who are drug responders and those who are non-responders, and to predict and propose likely effective treatments by machine, in the future.
The biggest challenge for the “SWIFT system” resides in “how to increase its accuracy.” The ultimate goal is to obtain sufficient accuracy to ensure adequate utility as a medical device. Sumitomo Dainippon Pharma plans to continue with long-term efforts for upgrading the product, since clinical research for increased data reliability takes a long time, and relevant systems need to be built at the same time.
In addition, at the Frontier Business Office of Sumitomo Dainippon Pharma Co., Ltd., we are endeavoring to conduct research and development for new values and business by combining our knowledge that has been compiled through R&D activities for pharmaceuticals for neuropsychiatric diseases and unique technologies, findings, and patents of our business partners. If you are interested in collaboration as our business partner, please contact us.
< Data Sources >
- 1)Kawakami N: Large-Scale Epidemiological Study on Prevalence Rates, etc., in Psychiatric Diseases: 2nd General Report of the World Mental Health Japan Survey (in Japanese), 2016.
- 2)Keio Gijuku Incorporated Educational Institution: Project Results Report for the Fiscal 2010 MHLW Project on General Promotion of the Welfare of Disordered Persons, Titled “Estimation of Social Cost from Psychiatric Diseases.”